Background
The current COVID-19 pandemic is caused by a coronavirus named SARS-CoV-2. Coronaviruses (CoVs) are a large family of viruses, several of which cause respiratory diseases in humans, from the common cold to more rare and serious diseases such as the Severe Acute Respiratory Syndrome (SARS) and the Middle East respiratory syndrome (MERS), both of which have high mortality rates and were detected for the first time in 2003 and 2012, respectively. SARS-CoV-2 is a positive-sense single-stranded RNA virus.
CoVs are divided into four genera: alpha-, beta-, gamma- and delta-CoV. All CoVs currently known to cause disease in humans belong to the alpha- or the beta-CoV. Many of these CoVs can infect several animal species as well. SARSCoV infected civet cats and infected humans in 2002 and MERS-CoV is found in dromedary camels and infected humans in 2012. A virus that is regularly transmitted from an animal to a human is called a zoonotic virus. When a virus passes from animals to humans for the first time it is called a spillover event.
Currently, the zoonotic source of SARS-CoV-2 is unknown. The first human cases of COVID-19, the coronavirus disease caused by SARS-CoV-2, were first reported from Wuhan City, China, in December 2019.
All SARS-CoV-2 isolated from humans to date are closely related genetically to coronaviruses isolated from bat populations, specifically, bats from the genus Rhinolophus. SARS-CoV, the cause of the SARS outbreak in 2003, is also closely related to coronaviruses isolated from bats. The analysis of the virus genome sequences indicates that SARS-CoV-2 is very welladapted to human cell receptors, which enables it to invade human cells and easily infect people.
Genome of SARS-CoV-2
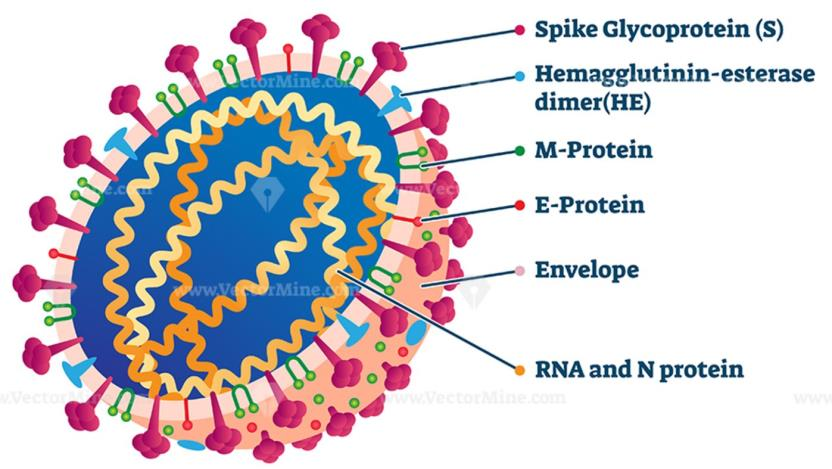
The genome of SARS-CoV-2 is approximately 30 kb in length and consists of six open reading frames (ORFs), which includes ORF1a/b, spanning 16 non-structural proteins (nsp) relating to the replicationtranscription complex, four structural proteins, spike (S), envelope (E), membrane (M), and nucleocapsid (N), along with several other nonstructural, special structural, and/or accessory ORFs (ORF3a/b, 6, 7a, 7b, 8, and 10)
Most diagnostic tests target a combination of structural (S, N, and/ or E) and non-structural (ORF1ab region) SARS-CoV-2 genes, along with positive and negative controls. This testing strategy ensures that the diagnostic targets include a nonstructural protein, highly conserved for coronaviruses, as well as structural protein(s), highly specific for SARS-CoV-2.
E / N gene assay as first-line screening assay and RdRp (RNA-dependent RNA polymerase) / ORF1ab gene assay as confirmatory assay. Alternatively, laboratories may choose to run the RdRp assay with only the 2019-nCoVspecific probe.
Symptoms of Coronavirus
COVID-19 affects different people in different ways. Most infected people will develop mild to moderate illness and recover without hospitalization.
Most common symptoms: fever, dry cough, tiredness.
Less common symptoms: aches and pains, sore throat, diarrhea, conjunctivitis, headache, loss of taste or smell, a rash on skin, or discoloration of fingers or toes.
Serious symptoms: difficulty breathing or shortness of breath, chest pain or pressure, loss of speech or movement.
On average it takes 5–6 days from when someone is infected with the virus for symptoms to show, however it can take up to 14 days.
How does COVID-19 spread?
The virus can spread from an infected person’s mouth or nose in small liquid particles when they cough, sneeze, speak, sing or breathe. These particles range from larger respiratory droplets to smaller aerosols. It spreads mainly between people who are in close contact with each other, typically within 1 meter (short-range).
A person can be infected when aerosols or droplets containing the virus are inhaled or come directly into contact with the eyes, nose, or mouth.
Laboratory Diagnosis
The gold standard for diagnosis is the identification of viral genome targets by real-time polymerase chain reaction (RT-PCR) in respiratory tract materials during the first week of symptoms. Serological tests should be indicated from the second week of symptoms onwards.
The antigen-detecting rapid diagnostic tests are sensitive only for detection of patients with a high viral load e.g. cycle threshold (Ct) values ≈25.
There is high chance that a negative rapid test could be a positive RT-PCR test and early detection can save the lives and helps disease management.
Recommendation: RT-PCR test
COVID – 19 Testing at Ruha Life Sciences
Sampling: respiratory material such as nasopharyngeal and / or oropharyngeal swab sampling is collected in a viral transport medium (VTM).
RT-PCR Molecular Testing: A Nucleic Acid Amplification Test, or NAAT, is a type of viral diagnostic test for SARS-CoV- 2, the virus that causes COVID-19. NAAT for SARS-CoV-2 specifically identify the RNA (ribonucleic acid) sequences that comprise the genetic material of the virus from the nasal and oral swab sampling in viral transport media (VTM). This test takes about 2 – 3 hours.
Rapid Testing: A rapid qualitative immunoassay for the nucleocapsid protein antigen of SARS-CoV-2 virus in human nasopharyngeal swab specimen. This test takes about 30 minutes. Presumptive positive or negative result may need to be further confirmed with a RT-PCR molecular test.
Note: A single negative test result, particularly if this is from an upper respiratory tract specimen, does not exclude infection. Repeat sampling and testing, lower respiratory specimen is strongly recommended in severe or progressive disease. A positive alternate pathogen does not necessarily rule out the role of coinfections.
CAUTION: Positive result indicates presence of SARS-CoV-2, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status.
Subscribe to our Newsletter
to receive up-to-date information
on new products and special offers




